Validation
If requested we can deliver completely validated ultra-pure water systems, which is quite common for projects in pharmaceutical industries.
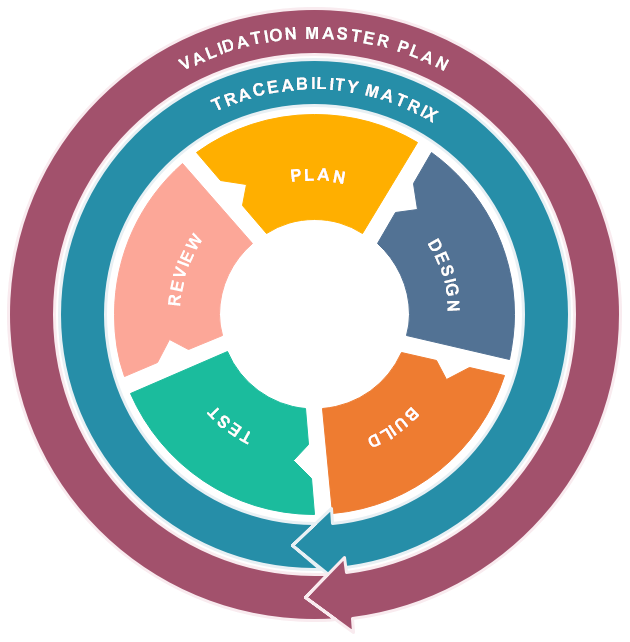
Validation is intended to ensure the project results in a product, service, or system that meets the operational needs of the user.
Therefore, in such projects, we will together with the customer make several documents and perform several checks and inspections.

We sell products from




The validation process we use, generally consist of the following phases:
- Design Qualification (DQ)
- confirming through review and testing that the equipment meets the written acquisition specification
- Installation Qualification (IQ)
- Inspection and checking of equipment and instrumentation
- Do the delivered components correspond with what was designed and ordered?
- Calibration of instrumentation where needed
- Certification of all components with IQ-sheets after installation
- Operation Qualification (OQ)
- Testing of equipment and instrumentation
- Is all equipment and instrumentation working as designed?
- Certification of all components with OQ-sheets after commissioning
- Performance Qualification (PQ)
Questions about our products?
Testing and reporting for a certain period to conclude that:
- the system operates as it was meant to
- the quality requirements are met
- the maintenance procedures are adequate to control the process
- Process Qualification (validation)
The process qualification starts when the PQ has proven that the process is working and the water is ready to be used. In this phase data should be collected on a continuous basis to support the initial validation data.